Breakthrough in Huntington’s disease treatment
One of the cruellest and most devastating illnesses – Huntington’s – has been successfully treated for the first time, according to doctors. The breakthrough Huntington’s disease treatment slowed disease progression in patients by 75%.
The disease runs through families, relentlessly kills brain cells and resembles a combination of dementia, Parkinson’s and motor neurone disease.
An emotional research team became tearful as they described how data shows the disease was slowed by 75% in patients.
It means the decline you would normally expect in one year would take four years after treatment, giving patients decades of “good quality life”, Prof Sarah Tabrizi told BBC News.
The new Huntington’s disease treatment is a type of gene therapy given during 12 to 18 hours of delicate brain surgery.
Life expectancy and early intervention
The first symptoms of Huntington’s disease tend to appear in your 30s or 40s and is normally fatal within two decades – opening the possibility that earlier treatment could prevent symptoms from ever emerging.

Prof Tabrizi, director of the University College London Huntington’s Disease Centre, described the results as “spectacular”.
“We never in our wildest dreams would have expected a 75% slowing of clinical progression,” she said.
None of the patients who have been treated are being identified, but one was medically retired and has returned to work. Others in the trial are still walking despite being expected to need a wheelchair.
Treatment is likely to be very expensive. However, this is a moment of real hope in a disease that hits people in their prime and devastates families.
Families living with Huntington’s

Huntington’s runs through Jack May-Davis’ family. He has the faulty gene that causes the disease, as did his dad, Fred, and his grandmother, Joyce.
Jack said it was “really awful and horrible” watching his dad’s inexorable decline.
The first symptoms appeared in Fred’s late 30s, including changes in behaviour and the way he moved. He eventually needed 24/7 palliative care before he died at the age of 54, in 2016.
Jack is 30, a barrister’s clerk, newly engaged to Chloe and has taken part in research at UCL to turn his diagnosis into a positive.
But he’d always known he was destined to share his father’s fate, until today.
Now he says the “absolutely incredible” breakthrough has left him “overwhelmed” and able to look to a future that “seems a little bit brighter, it does allow me to think my life could be that much longer”.
How Huntington’s disease develops
Huntington’s disease is caused by an error in part of our DNA called the huntingtin gene.
If one of your parents has Huntington’s disease, there’s a 50% chance that you will inherit the altered gene and will eventually develop Huntington’s too.
This mutation turns a normal protein needed in the brain – called the huntingtin protein – into a killer of neurons.
The goal of the treatment is to reduce levels of this toxic protein permanently, in a single dose.
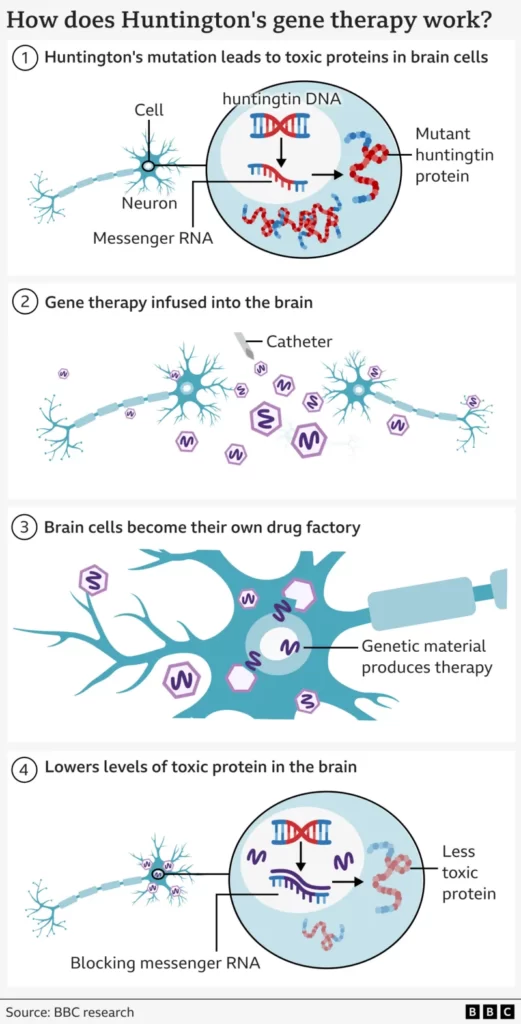
The new therapy approach
The therapy uses cutting edge genetic medicine combining gene therapy and gene silencing technologies.
It starts with a safe virus that has been altered to contain a specially designed sequence of DNA.
This is infused deep into the brain using real-time MRI scanning to guide a microcatheter to two brain regions – the caudate nucleus and the putamen. This takes 12 to 18 hours of neurosurgery.
The virus then acts like a microscopic postman – delivering the new piece of DNA inside brain cells, where it becomes active.
This turns the neurons into a factory for making the therapy to avert their own death.
The cells produce a small fragment of genetic material (called microRNA) that is designed to intercept and disable the instructions (called messenger RNA) being sent from the cells’ DNA for building mutant huntingtin.
This results in lower levels of mutant huntingtin in the brain.
Clinical trial results
Results from the trial – which involved 29 patients – have been released in a statement by the company uniQure, but have not yet been published in full for review by other specialists.
The data showed that three years after surgery there was an average 75% slowing of the disease based on a measure which combines cognition, motor function and the ability to manage in daily life.
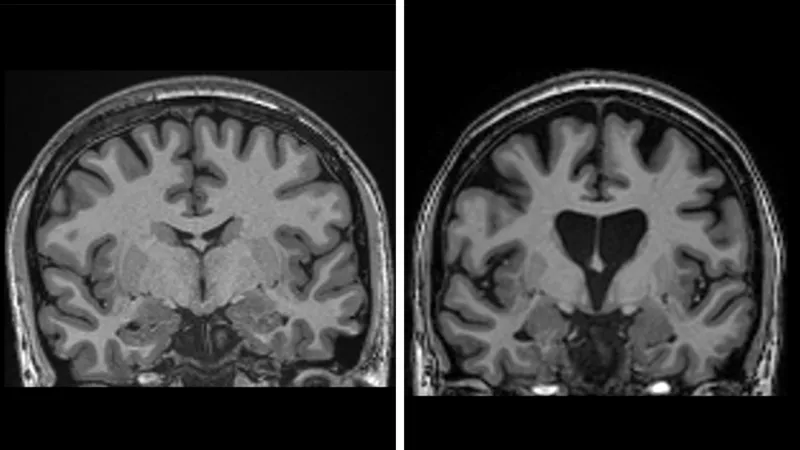
The data also shows the treatment is saving brain cells. Levels of neurofilaments in spinal fluid – a clear sign of brain cells dying – should have increased by a third if the disease continued to progress, but was actually lower than at the start of the trial.
“This is the result we’ve been waiting for,” said Prof Ed Wild, consultant neurologist at the National Hospital for Neurology and Neurosurgery at UCLH.
“There was every chance that we would never see a result like this, so to be living in a world where we know this is not only possible, but the actual magnitude of the effect is breathtaking, it’s very difficult to fully encapsulate the emotion.”
He said he was “a bit teary” thinking about the impact it could have on families.
The treatment was considered safe, although some patients did develop inflammation from the virus that caused headaches and confusion that either resolved or needed steroid treatment.
Prof Wild anticipates the therapy “should last for life” because brain cells are not replaced by the body in the same manner as blood, bone and skin are constantly renewed.
Next steps for approval
Approximately 75,000 people have Huntington’s disease in the UK, US and Europe with hundreds of thousands carrying the mutation meaning they will develop the disease.
UniQure says it will apply for a licence in the US in the first quarter of 2026 with the aim of launching the drug later that year. Conversations with authorities in the UK and Europe will start next year, but the initial focus is on the US.
Dr Walid Abi-Saab, the chief medical officer at uniQure, said he was “incredibly excited” about what the results mean for families, and added that the treatment had “the potential to fundamentally transform” Huntington’s disease.
However, the drug will not be available for everyone due to the highly complex surgery and the anticipated cost.
“It will be expensive for sure,” says Prof Wild.
There isn’t an official price for the drug. Gene therapies are often pricey, but their long-term impact means that can still be affordable. In the UK, the NHS does pay for a £2.6m-per-patient gene therapy for haemophilia B.
Looking ahead with hope
Prof Tabrizi says this gene therapy “is the beginning” and will open the gates for therapies that can reach more people.
She paid tribute to the “truly brave” volunteers who took part in the trial, saying she was “overjoyed for the patients and families”.
She is already working with a group of young people who know they have the gene, but don’t yet have symptoms – known as stage zero Huntington’s – and is aiming to do the first prevention trial to see if the disease can be significantly delayed or even stopped completely.
Source: BBC
Also read: ON THIS DAY: International Huntington’s Disease awareness day
For more videos and updates, check out our YouTube channel.


